ForsLean® is clinically proven to effect enhanced lean body mass and support healthy body composition. Epidemiological studies indicate that the impact of body composition on health starts early in life.
In a separate study, it was found that being overweight in adolescence predicted a broad range of adverse health effects that were independent of adult weight after 55 years of follow-up. Forskolin is known as a compound with versatile biological actions based on its ability to stimulate adenylate cyclase and cyclic AMP levels.
Adenylate cyclase is the enzyme involved in the production of Cyclic Adenosine Monophasphate (cAMP), (a significant biochemical agent that participates in metabolic processes), from the high energy molecule, ATP(Adenosine triphosphate). Nicknamed in scientific literature as a “second messenger”, cyclic AMP facilitates the action of “primary messengers” or various hormonal and bioactive substances in the body. The biochemical mechanism of maintaining or increasing lean body mass is related to the availability of cyclic AMP. By facilitating hormonal action, cyclic AMP may regulate the body’s thermogenic response to food, increase the body’s basic metabolic rate, and increase utilization of body fat (since thermogenesis is preferentially fueled by fatty acids derived from body fat and/or food). Typically, an increase in cyclic AMP leads to subsequent activation of protein kinase. Protein kinase has been shown to activate the hormone sensitive lipase which is involved in the breakdown of triglycerides, known as building blocks of fatty tissue.
Forskolin may also be involved in regulating insulin secretion. Insulin, although well recognized for its metabolism of carbohydrates, is also involved in the metabolism of fats and proteins that are major contributors to body composition.
Clinical studies in India, Japan and USA have revealed the efficacy of ForsLean® in reducing body weight and body fat and thereby enhancing lean body mass.
ForsLean® study abstracts
Badmaev V, Majeed M, Conte A, Parker JE (2000)
Diterpene forskolin (Coleus forskohlii, Benth.): A Possible new compound for reduction of body weight by increasing lean body mass
An extract of Coleus forskohlii root, Benth. (Fam. Labiatae) standardized for diterpene forskolin (ForsLean®) was tested in an open-field study for weight loss and lean body mass increase. The study’s hypothesis was based on the recognized role of diterpene forskolin as a plant derived compound, which stimulates enzyme adenylate cyclase and subsequently cyclic AMP (3’5’adenosine monophosphate). Cyclic AMP may release fatty acids from the adipose tissue depots which may result in enhanced thermogenesis, loss of body fat, and theoretically increased lean body mass.
Six overweight, but otherwise healthy, women were selected for the trial. Each participant was informed about the purpose of the study and was asked to sign an informed consent before entering the study. Each participant was examined by a physician at the inception and after 4 and 8 weeks of the study. Their body composition was determined by bioelectrical impedance analysis. ForsLean was prepared in the form of two-piece hard shell capsules. Each capsule contained 250 mg of the extract standardized for 10% forskolin. Participants were instructed to take one capsule in the morning and one in the evening, half an hour before a meal. Participants were asked to maintain their previous daily physical exercise and eating habits. In addition, physical activity was monitored based on a questionnaire before and during the trial. The study was performed in an outpatient bariatric clinic at Hilton Head, S.C. and supervised by a physician specializing in bariatric medicine for over 30 years.
During the eight week trial the mean values for body weight, and fat content were significantly decreased, whereas lean body mass was significantly increased as compared to the baseline (Wilcoxon matched pairs test). Weight loss was statistically significant (p<0.05) after 4 and 8 weeks, and the mean amounted to 4.3 and 9.17 lbs respectively. The body fat values expressed as % body fat were: 0 weeks 33.63 ± 3.02, 4 weeks 30.10 ± 4.34 (statistically not significant or n.s.), and 8 weeks 25.88 ± 4.77 (p<0.05). The lean body mass values expressed as % lean body mass were: 0 weeks 67.07 ± 3.02, 4 weeks 69.90 ± 4.34 (n.s.), and 8 weeks 74.13 ± 4.77 (p<0.05).
The eight-week therapy with 50 mg of forskolin per day did not adversely affect the systolic/diastolic blood pressure or the pulse rate. However, a trend was observed to lower the systolic/diastolic pressure in the course of treatment. Systolic pressure (mm Hg) was 0 weeks 113.67 ± 14.50, 4 weeks 110.00 ± 18.93 (n.s.), and 8 weeks 104.50 ± 17.54(n.s.). Diastolic pressure (mmHg) was 0 weeks 71.00 ± 12.76, 4 weeks 69.33 ± 9.93 (n.s.), and 8 weeks 66.00 ± 8.49(n.s.). Pulse rate (beats/min) was 0 weeks 66.33 ± 8.02, 4 weeks 69.00 ± 7.97(n.s.), and 8 weeks 74.67 ± 11.55(n.s.).
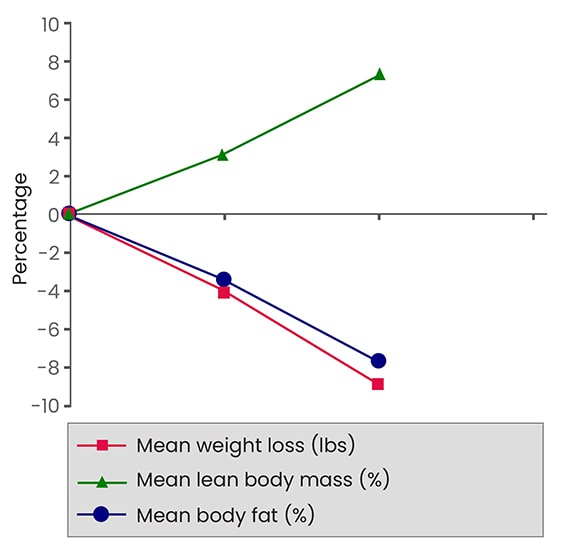
Open field study, 6 overweight women subjects, 500 mg ForsLean® corresponding to 50 mg forskolin/day for 8 weeks
This preliminary data obtained with 250 mg bid of ForsLean® 10% extract indicate that this composition bears promise as a safe and effective weight loss regimen. The effect of ForsLean® is particularly valid in the absence of change in frequency and intensity of physical exercise and without diet restrictions during the course of the trial. This study warrants a double-blind clinical trial evaluating effects of forskolin on body composition and its possible thermogenic mechanism.
Asano Tsuguyoshi (2001)
Clinical report on root extract of Coleus forskohlii ForsLean® in reducing body fat, Asano Institute. Tokyo, Japan
A standardized extract of Coleus forskohlii roots known as ForsLean® (10% diterpene forskolin) was evaluated in a 12 week open field study in overweight volunteers, 1 male and 13 females; average weight 74.7 ± 11.98 kg, average BMI 29.9 ± 4.31 and average body fat 38.2 ± 4.87%. ForsLean® was administered in a dose of 125 mg twice a day. Total daily intake of ForsLean® was calculated as 25 mg of diterpene forskolin. Each patient was examined in the physician’s office and body composition measurements were taken with an infrared analyzer Futurex 6200 on day 0, first month, second month and third month. Total body weight showed tendency to decrease from an average 74.7 kg at the onset of the study to 73.5 kg on the third month (p<0.05). Body mass index improved from an initial average value of 29.9 to 29.4 (p<0.05) at conclusion of the study. The body fat was decreased from an initial average value of 38.2% to 37.1% (p<0.01) at conclusion of the study. Lean body mass was preserved in the course of 12 week ForsLean® administration (average 45.8 kg vs. 45.9 kg). The 12-week regimen with 25 mg of forskolin per day did not significantly change blood pressure parameters, i.e. average systolic blood pressure 135.7 mmHg vs. 128 mmHg; average diastolic blood pressure 85.3 mmHg vs. 83.6 mmHg. This 12 week open field study of ForsLean® on 14 overweight Japanese subjects indicates its usefulness in weight loss management with no apparent subjective and objective side effects of the regimen.
Krieder R et al., (2002)
Effects of Coleus forskohlii extract supplementation on body composition and markers of health in sedentary overweight female
In a double blind and randomized manner, 23 females supplemented their diet with ForsLean®(250 mg of 10% Coleus forskohlii [CF] extract, n=7) or a placebo (n=12) two times per day for 12 weeks. Body composition (DEXA), body weight, and psychometric instruments were obtained at 0, 4, 8 & 12 weeks of supplementation. Fasting blood samples and dietary records (4-d) were obtained at 0 and 12 weeks. Side effects were recorded on a weekly basis. Data were analyzed by repeated measures ANOVA and are presented as mean changes from baseline for the CF and placebo groups, respectively. No significant differences were observed in caloric or macronutrient intake. CF tended to mitigate gains in body mass (-0.7 ± 1.8, 1.0 ± 2.5 kg, p=0.10) and scanned mass (-0.2 ± 1.3, 1.7 ± 2.9 kg, p=0.08) with no significant differences in fat mass (-0.2 ± 0.7, 1.1 ± 2.3 kg, p=0.16), fat free mass (-0.1 ± 1.3, 0.6 ± 1.2 kg, p=0.21), or body fat (-0.2 ± 1.0, 0.4 ± 1.4 %, p=0.40). Subjects in the CF group tended to report less fatigue (p=0.07), hunger (p=0.02), and fullness (p=0.04). No clinically significant interactions were seen in metabolic markers, blood lipids, muscle and liver enzymes, electrolytes, red cells, white cells, hormones (insulin, TSH, T3, and T4), heart rate, blood pressure, or weekly reports of side effects. Results suggest that CF may help mitigate weight gain in overweight females with apparently no clinically significant side effects.
Bhagwat, A.M. et al., (2004)
A Randomized Double-Blind Clinical Trial to Investigate The Efficacy and Safety of ForsLean in Increasing Lean Body Mass
Shri C. B. Patel Research Center for Chemistry and Biological Sciences, Mumbai, India.
In a 12 week double blind and randomized study, 60 obese male and female volunteers, 25 – 45 years old with a Body Mass Index (BMI) between 28 – 40 and / or body fat concentration above 30% in males and 40% in females received 25 mg of diterpene forskolin bid in the form of ForsLean® (250 mg of 10% Coleus forskohlii roots extract) or a matching placebo. The volunteers receiving the ForsLean® on average lost 3.81 pounds or 2.3% of their total body weight, while the placebo group gained an average one half pound or 0.3% of the total body weight. The volunteers treated with the placebo gained 0.32% of body fat, while the ForsLean® receiving group lost 0.87% of body fat. The difference in the weight and fat reduction was statistically significant between the active and placebo groups. The thyroid function tests were performed, assessing levels of hormones T3, T4 and TSH before and after the completion of the study. It was observed that the levels of all three hormones remained within normal range in both active and placebo treated groups after 12 weeks of the regimen. The blood lipid profile, performed at the onset and at the conclusion of the study, included triglycerides, total cholesterol, HDL, LDL and VLDL. Ratio of total cholesterol to HDL was also calculated. At the end of 12 weeks, the placebo treated volunteers did not show any significant change in any of the lipid parameters recorded. However, those volunteers on the active compound showed a significant rise in the concentrations of HDL at the end of the study, while triglycerides, total cholesterol, LDL and VLDL levels remained unchanged in this group as compared to baseline and placebo group levels.
In conclusion, based on this 12 week clinical study ForsLean® may have a weight and fat reduction property. In addition, as compared with the placebo receiving group, ForsLean® may help preserve lean body mass. The 12-week treatment did not produce any subjective or objective side effects in either the active compound or placebo-receiving group. The laboratory data indicate that ForsLean® regimen did not alter the thyroid hormones and blood lipid profile, with exception of increase in the HDL serum levels and significant decrease of total cholesterol/HDL ratio as compared to the control group.
Kamath, M.S. et al., (2004)
Efficacy and safety in increasing lean body mass in class I obese subjects, Kasturba Medical College, Manipal, India
50 subjects, male and female, were randomized to receive 250 mg of ForsLean® or Placebo capsules twice a day (morning and evening) half an hour before meals for 12 weeks. Significant decrease in body weight and fat content and significant increase in lean body mass was observed.
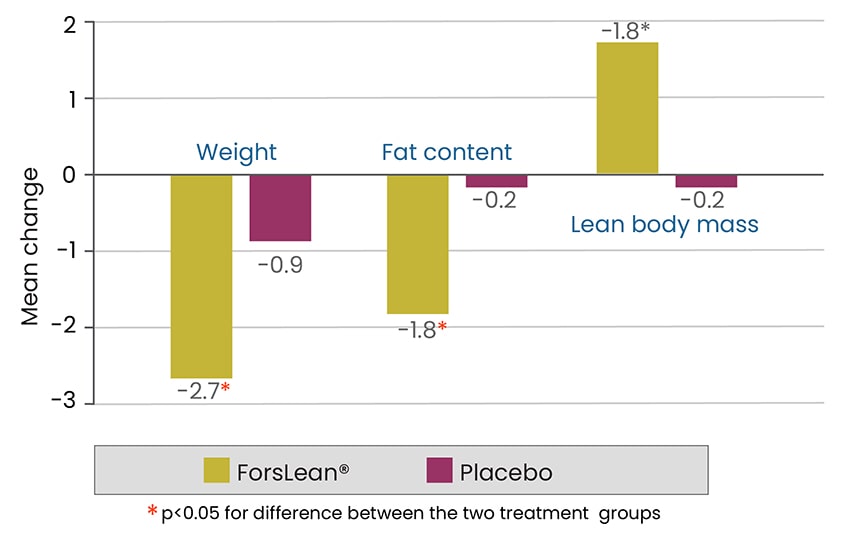
Mean change in lean body mass, fat content and body weight (n=50)
